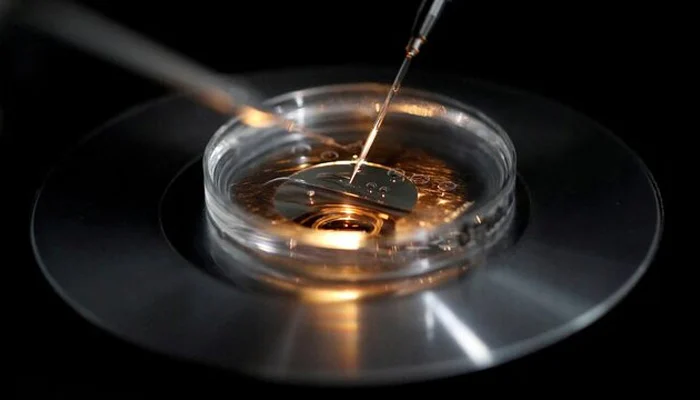
Scientists from Newcastle University reported on Wednesday that eight children in the UK have been successfully protected from severe genetic diseases through a pioneering three-person in vitro fertilization (IVF) technique. This innovative method, which is currently prohibited in the United States, involves transferring the nucleus from the mother’s fertilized egg, along with the nucleus of the father’s sperm, into a healthy egg provided by an anonymous donor.
The procedure’s core benefit is its ability to prevent the transmission of mutated genes found within the mother’s mitochondria—the cellular “powerhouses”—which can otherwise lead to incurable and potentially fatal disorders. Mutations in mitochondrial DNA can adversely affect multiple organs, particularly those with high energy demands such as the brain, liver, heart, muscles, and kidneys.
Of the eight children, one is now two years old, two are between one and two years old, and five are infants. The scientists, reporting in the New England Journal of Medicine, confirmed that all children were healthy at birth, with blood tests indicating either no or very low levels of mitochondrial gene mutations. They also noted that all eight children have shown normal developmental progress.
Dr. Andy Greenfield, a reproductive medicine specialist at the University of Oxford not involved in the research, commented in a statement that these results “are the culmination of decades of work.” He emphasized that this effort encompassed not only scientific and technical challenges but also extensive ethical inquiry, public and patient engagement, law-making, the drafting and execution of regulations, and the establishment of a system for monitoring and caring for both mothers and infants. Greenfield further stated that the researchers’ “treasure trove of data” is likely to serve as a crucial starting point for new avenues of investigation.
Typically, during standard IVF screening procedures, doctors can identify some low-risk eggs with minimal mitochondrial gene mutations suitable for implantation. However, there are instances where all of the mother’s eggs carry mitochondrial DNA mutations. In such cases, using this new technique, UK doctors first fertilize the mother’s egg with the father’s sperm. They then carefully remove the “pronuclei” from this fertilized egg—these are the nuclei of both the egg and the sperm, containing the complete DNA instructions from both parents for the baby’s development, survival, and reproduction.
Next, these combined egg and sperm nuclei are transferred into a donated fertilized egg from which its own pronuclei have been removed. This newly constituted donor egg then begins to divide and develop, now featuring healthy mitochondria from the donor and the nuclear DNA from both the biological mother’s egg and the biological father’s sperm.
This process, detailed in a second paper published in the same journal, “essentially replaces the faulty mitochondrial DNA (mtDNA) with healthy mtDNA from the donor,” explained senior researcher Mary Herbert, a professor of reproductive biology at Newcastle, during a press briefing. The researchers reported in the second paper that blood levels of mtDNA mutations were significantly lower in the newborns: 95% to 100% lower in six infants, and 77% to 88% lower in two others, when compared to the levels of the same variants in their mothers. They concluded, “These data indicate that pronuclear transfer was effective in reducing transmission of mtDNA disease.”
The procedure was tested in 22 women whose babies were at risk of inheriting such genetic conditions. In addition to the eight women who have successfully delivered children, one of the remaining 22 women is currently pregnant. Seven of the eight pregnancies were uneventful, with only one pregnant woman showing high lipid levels in blood tests. Notably, there have been no reported miscarriages.
The authors of the current reports have also explored an alternative approach involving transplanting the nucleus of a mother’s unfertilized egg into a donor egg before fertilization. However, they believe their newly detailed method may offer a more reliable way to prevent the transmission of these genetic disorders.
In a landmark decision in 2015, the UK became the first country globally to legalize research into mitochondrial donation treatment for human use. In stark contrast, during the same year in the United States, pronuclear transfer for human use was effectively banned by a congressional appropriations bill that prohibited the Food and Drug Administration from using funds to consider the use of “heritable genetic modification.”